
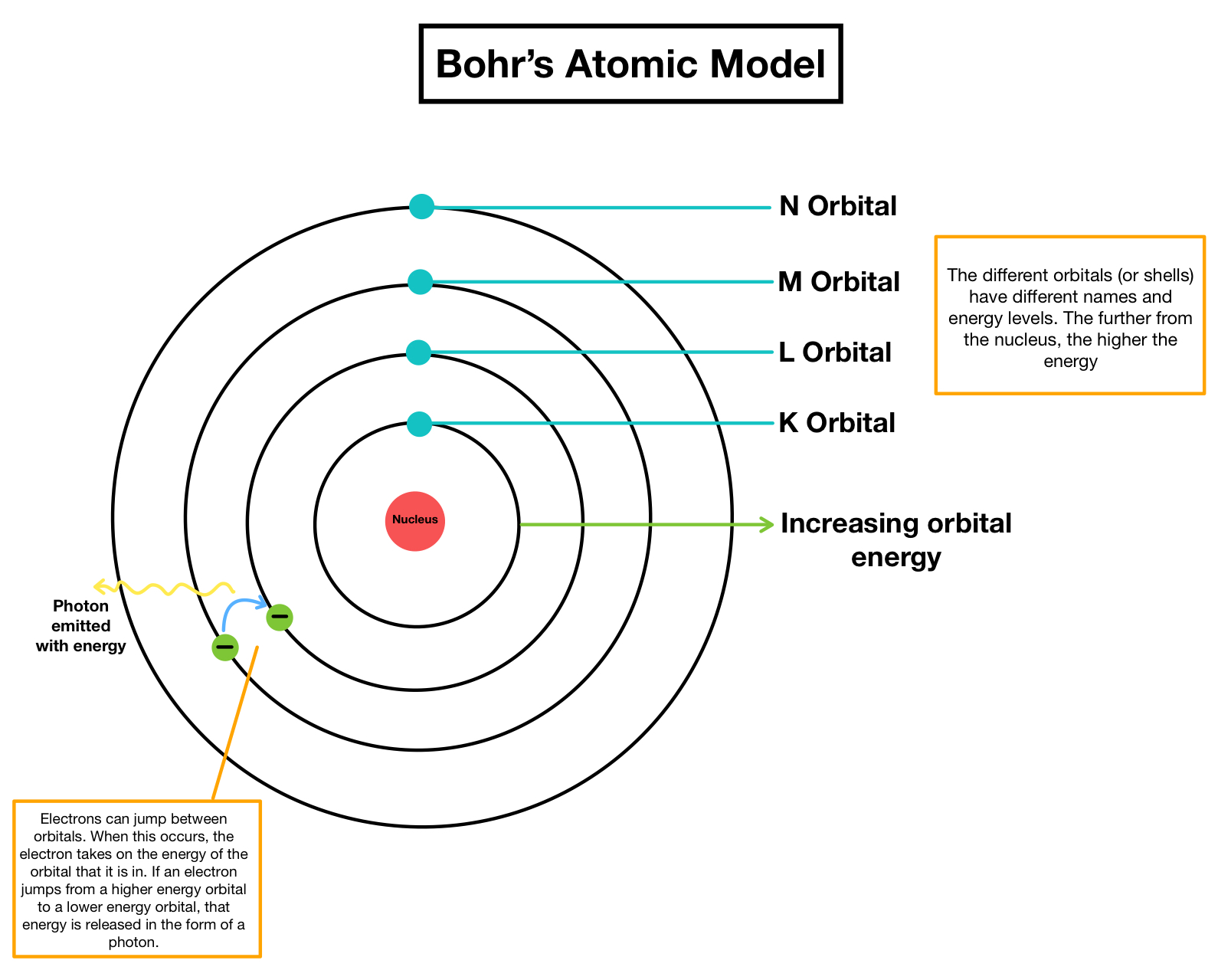

The neutron mass is almost similar to the proton mass. The five postulates of Daltons Atomic Theory are: All matter is made up of tiny, indivisible particles called atoms.He concluded that the nucleus contains another tiny particle known as a neutron that has no charge.Protons and neutrons form the atomic nucleus. The concepts of this foundation include the atomic theory, the composition and mass of an atom, the variability of the composition of isotopes, ion formation, chemical bonds in ionic and covalent compounds, the types of chemical reactions, and the naming of compounds. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Chadwick discovered the presence of neutrons in the nucleus. The atomic model, which has changed over time, is the model used to describe the structure and composition of the atom. An atom is a building block of matter that cannot be broken apart using any chemical means.In 1922, the American physicist Arthur H. Each orbit forms a circle and has a fixed distance from the nucleus. The development of quantum mechanics served as the foundation of the modern atomic theory.Electrons move around the nucleus in fixed orbits. The advantage of this model is that it consists of mathematical equations known as wave functions that satisfy the requirements placed on the behavior of.Electrons in an atom of an element are not randomly distributed around the atomic nucleus.Those alpha particles that had come into close proximity with the nucleus had been strongly deflected whereas the majority had passed at a relatively great distance to it.
The nucleus was around 10-15 meters in diameter, in the centre of a 10-10 metre diameter atom.most of the atom’s mass concentrated in a tiny center, the nucleus and electrons being held in orbit around it by electrostatic attraction. Today, scientists use an atomic model that has a central, positively-charged nucleus that contains: positively-charged protons.


 0 kommentar(er)
0 kommentar(er)
